b) pH = 10.97
c) [N2H62+] = 1.86x10-14 M
| Home / CHM 106 Summer 2005 / Problem Set 3 Solutions | Contact Information |
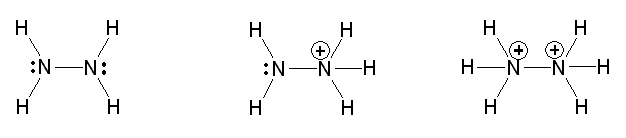
1a) 
b) pH = 10.97
c) [N2H62+] = 1.86x10-14 M
2a) 39.45 g NaOH (0.986 mol)
b) 5.96 g NaOH (0.149 mol)
c) Phosphoric acid buffer changes to pH = 3.76 (0.24 units), acetic acid buffer changes to pH = 3.96 (0.04 units)
d) Phosphoric acid buffer changes to pH = 4.54 (0.54 units), acetic acid buffer changes to pH = 4.03 (0.03 units)
e) The acetic acid / sodium acetate buffer has the highest capacity. Since the pH is closer to the pKa for acetic acid than phosphoric acid, the ratio of acetate to acetic acid will be closer to 1 than the ratio of dihydrogen phosphate to phosphoric acid. Thus, the acetic acid ratio will change less for any given amount of acid or base added than the phosphoric acid ratio will and it will be able to resist changes in pH better.
| Last modified 15 June 2005 | Copyright © 2005 Michael Shevlin |